There are millions of cars on the road and each one is potentially a source of air pollution. To aid in solving these pollution problems, cities, states and the Government create clean air laws that restrict the amount of pollution vehicles can produce. A catalytic converter can assist in reducing harmful gasses emitted by the vehicle’s engine.
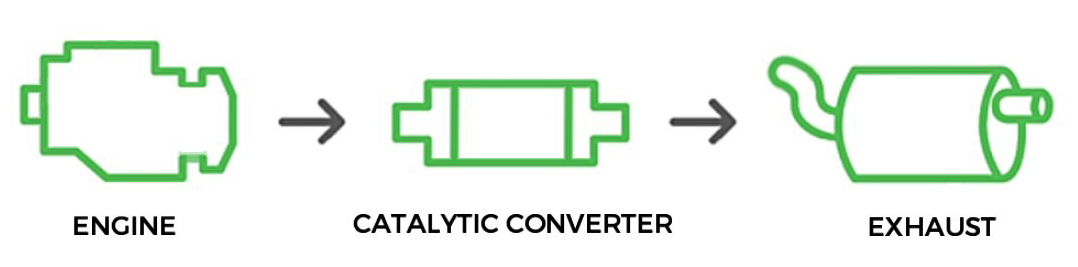
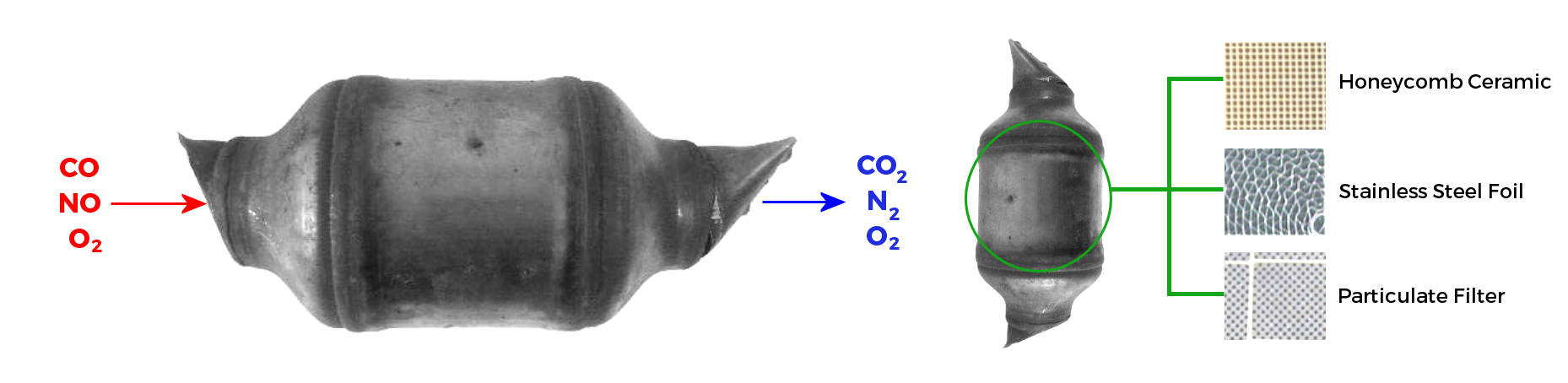
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalysing a redox reaction. Most modern cars are equipped with three-way catalytic converters. "Three-way" refers to the three regulated emissions it helps to reduce -carbon monoxide, VOCs and NOx molecules. The converter uses two different types of catalysts, a reduction catalyst, and an oxidization catalyst. Both types consist of a ceramic structure coated with a metal catalyst, usually platinum, rhodium and/or palladium. The idea is to create a structure that exposes the maximum surface area of catalyst to the exhaust stream, while also minimizing the amount of catalyst required.
There are two main types of structures used in catalytic converters, honeycomb and ceramic beads. Although catalytic converters are most commonly applied to exhaust systems in automobiles, they are also used on electrical generators, forklifts, mining equipment, trucks, buses, locomotives, motorcycles, and on ships, they can also come in varieties such as wash coat coated steel and particulate filter.